How does PMED™ work?
The core PMED technology comprises two distinct elements:
- The formulation of DNA therapeutic vaccines as stable, dry
powders of DNA precipitated onto the surface of microscopic
gold particles and contained within a cassette
- The gas-powered, single-use PMED device (Fig 1)
The DNA plasmid that forms the active component of the
therapeutic vaccine is precipitated onto microscopic gold
particles (typically 2µg DNA on 1mg gold). Microscopic elemental
gold particles (mean particle diameter 1 - 3 microns) are used
as the plasmid DNA carrier, because it is inert and has the
appropriate density needed to deliver the vaccine directly into
the target epidermal antigen-presenting cells (APCs). These
microscopic particles appear as a stable dry powder. This powder
is then filled into sealed cassettes. These cassettes act as the
primary drug product container and are inserted into the PMED
device during the final product assembly and packaging process.
The PMED device is a single-use, disposable device powered by
high pressure helium (Figure 1). The cassette containing the
powdered vaccine is loaded into the body of the device at the
end of the manufacturing process. As the powdered vaccine is
stable at ambient temperature and the cassette is sealed, PMED
can be stored simply and cheaply.
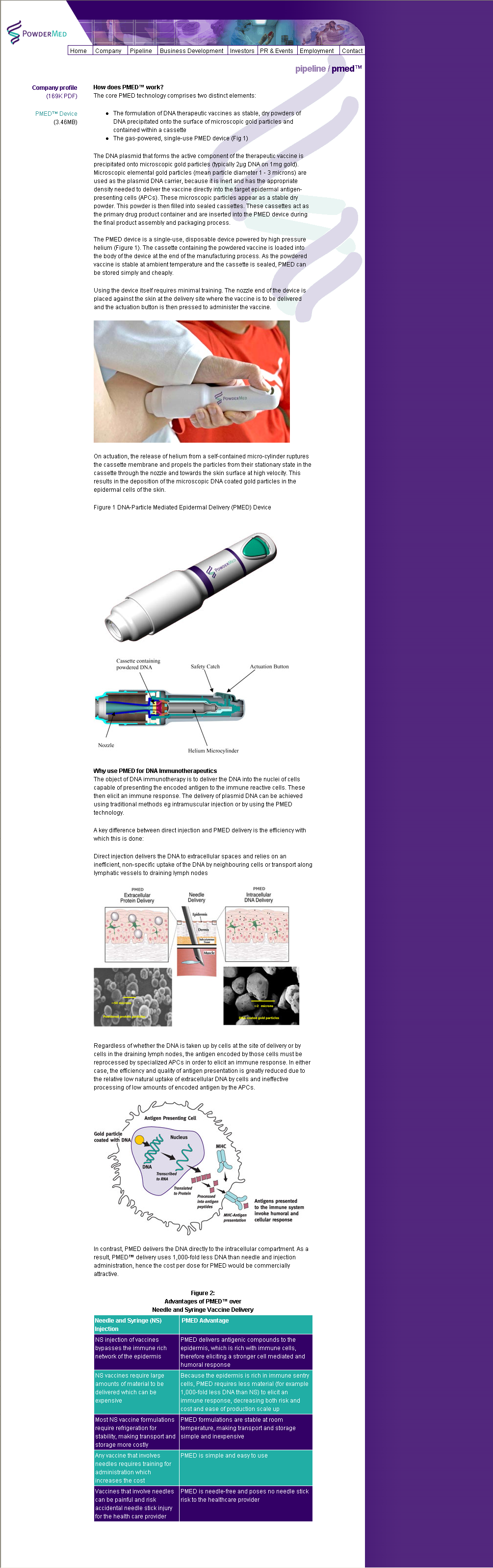
Using the device itself requires minimal training. The nozzle
end of the device is placed against the skin at the delivery
site where the vaccine is to be delivered and the actuation
button is then pressed to administer the vaccine.

On actuation, the release of helium from a self-contained
micro-cylinder ruptures the cassette membrane and propels the
particles from their stationary state in the cassette through
the nozzle and towards the skin surface at high velocity. This
results in the deposition of the microscopic DNA coated gold
particles in the epidermal cells of the skin.
Figure 1 DNA-Particle Mediated Epidermal Delivery (PMED)
Device


Why use PMED for DNA Immunotherapeutics
The object of DNA immunotherapy is to deliver the DNA into the
nuclei of cells capable of presenting the encoded antigen to the
immune reactive cells. These then elicit an immune response. The
delivery of plasmid DNA can be achieved using traditional
methods eg intramuscular injection or by using the PMED
technology.
A key difference between direct injection and PMED delivery
is the efficiency with which this is done:
Direct injection delivers the DNA to extracellular spaces and
relies on an inefficient, non-specific uptake of the DNA by
neighbouring cells or transport along lymphatic vessels to
draining lymph nodes

Regardless of whether the DNA is taken up by cells at the
site of delivery or by cells in the draining lymph nodes, the
antigen encoded by those cells must be reprocessed by
specialized APCs in order to elicit an immune response. In
either case, the efficiency and quality of antigen presentation
is greatly reduced due to the relative low natural uptake of
extracellular DNA by cells and ineffective processing of low
amounts of encoded antigen by the APCs.

In contrast, PMED delivers the DNA directly to the
intracellular compartment. As a result, PMED™
delivery uses 1,000-fold less DNA than needle and injection
administration, hence the cost per dose for PMED would be
commercially attractive.
|
Figure 2:
Advantages of PMED™ over
Needle and Syringe Vaccine Delivery
|
|
Needle and Syringe (NS) Injection |
PMED Advantage |
| NS
injection of vaccines bypasses the immune rich network of
the epidermis |
PMED
delivers antigenic compounds to the epidermis, which is rich
with immune cells, therefore eliciting a stronger cell
mediated and humoral response |
| NS
vaccines require large amounts of material to be delivered
which can be expensive |
Because the epidermis is rich in immune sentry cells, PMED
requires less material (for example 1,000-fold less DNA than
NS) to elicit an immune response, decreasing both risk and
cost and ease of production scale up |
| Most
NS vaccine formulations require refrigeration for stability,
making transport and storage more costly |
PMED
formulations are stable at room temperature, making
transport and storage simple and inexpensive |
| Any
vaccine that involves needles requires training for
administration which increases the cost |
PMED
is simple and easy to use |
|
Vaccines that involve needles can be painful and risk
accidental needle stick injury for the health care provider |
PMED
is needle-free and poses no needle stick risk to the
healthcare provider |
|